
Lab Staff
Research in the Gale laboratory is focused on understanding the processes that trigger and control innate immunity and inflammation to program the immune response against RNA virus infection, and to define the virus-host interactions that control viral replication and the outcome of infection and immunity. We are also focused on defining the systems biology and innate immune interactions of acute and chronic microbial infection toward building interventions to fight disease and improve global health.
The laboratory is a member of the CIIID and is a component of the Systems Immunogenics Consortium, the Immune Mechanisms of Protection Against Mycobacterium Tuberculosis (IMPAc-TB) consortium, the Adjuvant Discovery and Development Program, the Infectious Disease Clinical Research Consortium (IDCRC), and the United World Arbovirus Research Network (UWARN), each funded by the National Institutes of Health (NIH). We are a component of the consortium for Development and Advancement of Broad-spectrum Respiratory Antivirals (DABRA) supported by the Department of Defense. The lab is also a member of the HIV Reservoir consortium supported by the Bill and Melinda Gates Foundation. We operate the NIH-funded Nonhuman Primate Functional Genomics Core for AIDS vaccine Development. Additionally, The Gale laboratory has active research programs focused on understanding immune control of infection by hepatitis C virus, hepatitis B viruses, HIV, SIV, flaviviruses including Zika virus and West Nile virus, Hanta virus, SARS-CoV-2 and contemporary coronaviruses, and influenza viruses. We are also engaged in programs of study to understand the role of innate immunity and immune programming in maternal-fetal health. Our research team is working at the forefront of innate immunity to understand the immunomodulatory/antiviral actions of interferons, and to develop small molecule innate immune agonists as antiviral mediators for the clinical treatment of virus infection and as immune modulators to program the immune response. The lab works closely with collaborators within academic, biotechnology, and pharmaceutical institutions in Seattle, the USA, and across the world to conduct research to build new or improved vaccines and therapeutics to improve global health in the fight against SARS-CoV-2, HIV, Yellow Fever virus, West Nile virus, Zika virus, hepatitis B virus, and influenza A virus. We are a member lab of the UW Center for Emerging and Re-emerging Infectious Diseases (CERID) where we work to isolate and study novel emerging viruses. We are committed to teaching and training scientists to be educators, researchers, and clinicians in the areas of immunology, virology, public and global health, systems biology, and microbial infection and immunity.
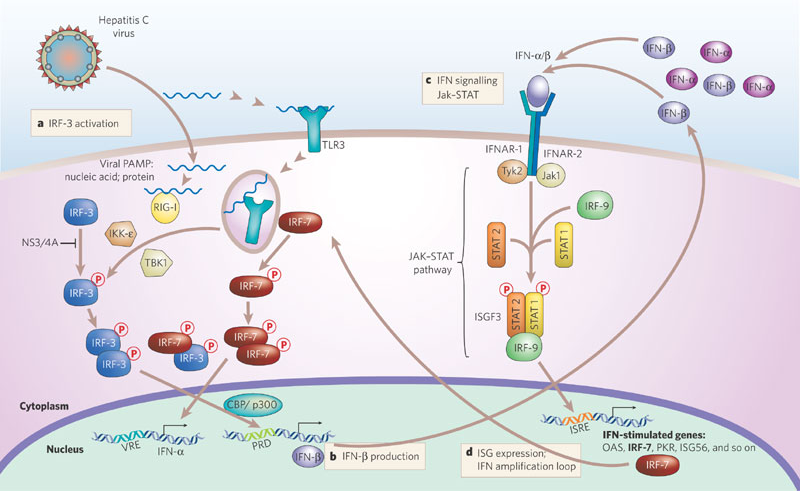
Description: The figure depicts hepatitis C virus.
Innate Immune signaling by RNA viruses. Michael Gale Jr. et al. (2005) Nature 436: 939-940)
 |
|
|
|
|
 |

